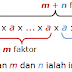
A multicellular organism is made up of millions of cells, all of which carry out specific and different functions depending on their shapes and structures, and the organelles contained in them.
In multicellular organisms, cells make up tissues, tissues make up organs and organs make up systems.
In an entity, they cooperate with one another and ensure the organism carry out its seven main living processes.
The kind of organisation is extremely necessary to allow different systems to carry out specific functions and to enable the organism to be a complete and functional individual.
Cell organisation
In multicellular organisms, cells make up tissues, tissues make up organs and organs make up systems.
In an entity, they cooperate with one another and ensure the organism carry out its seven main living processes.
The kind of organisation is extremely necessary to allow different systems to carry out specific functions and to enable the organism to be a complete and functional individual.
Cell organisation
- To gauge whether you know how multicellular organisms from the plant and animal kingdoms are organised, as the following table.
Plant kingdom
Level of organisation
Animal kingdom
Hibiscus plant
Organism
Man
Shoot system
System
Circulatory system
Leaf
Organ
Skin
Vascular tissue
Tissue
Connective tissue
Mesophyll palisade cell
Cell
Epithelial cell
- Another example of how two other multicellular organisms are organised.
Plant kingdom
Level of organisation
Animal kingdom
Maze
Organism
Rat
Root system
System
Circulatory system
Root
Organ
Lungs
Ground tissue
Tissue
Connective tissue
Parenchyma
Cell
Red blood cell