Tenaga yang boleh diperbaharui dan yang tidak boleh diperbaharui
-
Sumber-sumber tenaga yang terdapat di dunia ini adalah boleh dikelaskan
kepada dua kumpulan, iaitu:
1. Sumber tenaga yang boleh diperbaharui.
2. Sumb...
Friday, September 18, 2009
Tuesday, September 08, 2009
2 Sit UPSR Exam In Hospitals
NST
KUALA LUMPUR: Two students who are down with influenza A (H1N1) symptoms are among the 517,908 candidates nationwide who are sitting the Ujian Pencapaian Sekolah Rendah (UPSR) examination which begins today.
Education director-general Tan Sri Alimuddin Mohd Dom said preparations had been made for the students, one in Johor and the other in Penang, to sit the examination in hospitals.
He said these were among the measures taken to curb the spread of influenza A (H1N1) among UPSR candidates who were sitting the examination at 8,289 centres nationwide.
The Education Ministry had allocated about RM5 million for masks, hand sanitisers and thermometers for UPSR candidates, he said here yesterday.
He said an isolation room was available at all examination centres to accommodate those with a body temperature of above 37.5oC during screenings, which would be conducted upon their arrival at the centres. - Bernama
KUALA LUMPUR: Two students who are down with influenza A (H1N1) symptoms are among the 517,908 candidates nationwide who are sitting the Ujian Pencapaian Sekolah Rendah (UPSR) examination which begins today.
Education director-general Tan Sri Alimuddin Mohd Dom said preparations had been made for the students, one in Johor and the other in Penang, to sit the examination in hospitals.
He said these were among the measures taken to curb the spread of influenza A (H1N1) among UPSR candidates who were sitting the examination at 8,289 centres nationwide.
The Education Ministry had allocated about RM5 million for masks, hand sanitisers and thermometers for UPSR candidates, he said here yesterday.
He said an isolation room was available at all examination centres to accommodate those with a body temperature of above 37.5oC during screenings, which would be conducted upon their arrival at the centres. - Bernama
[Chemistry Form 4] Alkaline Aqueous
Hydroxides of sodium, potassium, magnesium and calcium are bases. Bases have many common features: they have a bitter taste, are slippery to the touch, change the colour of indicators, etc. Aqueous solutions of soluble bases are called alkalis.
Solution vs Ionisation
Some strong bases like calcium hydroxide aren't very soluble in water. That doesn't matter as what does dissolve is 100% ionised into calcium ions and hydroxide ions. Thus, calcium hydroxide still counts as a strong base because of that 100% ionisation.
*******
Hydroxide that are alkalis:
The following the dissociation of acids and base.
Hydrochloric acid
Formula: HCl
Equation to show dissociation: HCL -> H+ + Cl-
Nitric acid
Formula: HNO3
Equation to show dissociation: HNO3 -> H+ + NO3-
Ethanoic acid
Formula: CH3COOH
Equation to show dissociation: CH3COOH -> CH3COO- + H+
Potassium hydroxide
Formula: KOH
Equation to show dissociation: KOH -> K+ + OH-
Sodium hydroxide
Formula: NaOH
Equation to show dissociation: NaOH -> Na+ + OH-
Calcium hydroxide
Formula: Ca(OH)2
Equation to show dissociation: Ca(OH)2 -> Ca2+ + 2OH-
*******
Solution vs Ionisation
Some strong bases like calcium hydroxide aren't very soluble in water. That doesn't matter as what does dissolve is 100% ionised into calcium ions and hydroxide ions. Thus, calcium hydroxide still counts as a strong base because of that 100% ionisation.
*******
Hydroxide that are alkalis:
- NaOH
- Mg(OH)2
- KOH
- LiOH
- NH4OH
The following the dissociation of acids and base.
Hydrochloric acid
Formula: HCl
Equation to show dissociation: HCL -> H+ + Cl-
Nitric acid
Formula: HNO3
Equation to show dissociation: HNO3 -> H+ + NO3-
Ethanoic acid
Formula: CH3COOH
Equation to show dissociation: CH3COOH -> CH3COO- + H+
Potassium hydroxide
Formula: KOH
Equation to show dissociation: KOH -> K+ + OH-
Sodium hydroxide
Formula: NaOH
Equation to show dissociation: NaOH -> Na+ + OH-
Calcium hydroxide
Formula: Ca(OH)2
Equation to show dissociation: Ca(OH)2 -> Ca2+ + 2OH-
*******
Subscribe to:
Posts (Atom)
Nota Terkini
-
-
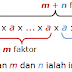
Hukum-hukum Indeks – Hukum 1 - *Hukum-hukum Indeks – Hukum 1* *am x** an= am+n* dimana *a*, *m* dan *n* adalah nombor nyata. *Pembuktian Hukum 1* *Contoh 1* Cari nilai bagi yang ber...
-
Tindakan Pencucian Sabun - Formula am sabun dapat diwakili dengan RCOONa untuk garam natrium atau RCOOK untuk garam kalium dengan R sebagai kumpulan alkil yang berantai panjang. Co...
-
Kepupusan Haiwan - *Kepupusan haiwan* (animal extinction) bermaksud *lenyapnya* atau *sudah tiada lagi* sesuatu haiwan itu di muka bumi ini. Kewujudan haiwan-haiwan tersebut...
-
Tenaga Kinetik & Halaju Elektron Dalam Tiub Sinar Katod - Apabila suatu voltan, *V*, dikenakan merentasi katod dan anod suatu senapang elektron, tenaga elektrik yang dibekalkan kepada setiap elektron ialah *eV*, d...
-
Penukaran Unit Isipadu Cecair dalam Perpuluhan dan Pecahan - *Menukar unit isipadu cecair (mililiter dan liter) dalam perpuluhan dan pecahan*. Hubungan antara unit isipadu cecair adalah seperti berikut: Untuk menuk...
-
Protein - *Protein* ialah sebatian organik kompleks yang terdiri daripada unsur *karbon*, *hidrogen*, *oksigen* dan *nitrogen*. Kebanyakan protein juga mengandungi u...
-
Kelenjar Endokrin - Bukan semua koordinasi aktiviti badan dikawal dan diselaraskan oleh sistem saraf. Sesetengahnya dikawal dan diselaraskan oleh *sistem endokrin*, contohnya...
-
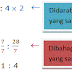
Menentukan sama ada nisbah yang diberi ialah nisbah setara - Nisbah bagi dua kuantiti tidak berubah jika kedua-dua nombor (kuantiti) dalam nisbah didarab atau dibahagi dengan faktor yang sama. Misalnya, *Contoh* ...
-
Menentukan Punca Persamaan Kuadratik Menggunakan Kaedah Pemfaktoran - Untuk menentukan punca persamaan kuadratik, kita menggunakan *kaedah pemfaktoran* (factorisation method), iaitu (*x* + *p*)(*x* + *q*) = 0 atau ( *mx* + *p...