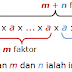
- Anaphase II
- The sister chromatids are pulled apart and head towards opposite ends of the cell.
- The sister chromatids are pulled apart and head towards opposite ends of the cell.
- Prophase I
- The homologous chromosomes, which are visible under a microscope, appear short, dense and thick. Each chromosome forms its own set of sister chromatids. The homologous chromosomes exist as two tetrads with a total of eight chromatids altogether. Crossing over may occur between non-sister chromatids of the homologous chromosomes.
- Telophase I
- Spindle fibres disappear. The cell divides into two, each having two chromosomes each. The sister chromatids for each homologous chromosome are still present and attached to a common centromere.
- Telophase II
- Each end of the cell now has two chromosomes. The nucleoli and nuclear membranes re-form. This stage is followed by cytokinesis.
- Anaphase I
- The homologous chromosomes separate and move towards opposite ends of the cells.
- Prophase II
- There are now two chromosomes, each with their sister chromatids present, in each of the two haploid cells. The chromatids thicken and shorten. The nuclear membrane around them disappears again and the chromatids are free to move.
- Metaphase II
- The sister chromatids align themselves at the imaginary centre of the cell.
- Metaphase I
- The homologous chromosomes, together with their chromatids, align themselves at an imaginary line along the centre of the cell.
- The homologous chromosomes, together with their chromatids, align themselves at an imaginary line along the centre of the cell.
English ~ Bahasa Melayu
anaphase ~ anafasa
cell division ~ pembahagian sel
chromosome ~ kromosom
cytokinesis ~ sitokinesis
daughter cell ~ sel anak
interphase ~ interfasa
metaphase ~ metafasa
parent cell ~ sel induk
prophase ~ profasa
telophase ~ telofasa
***